Adaptive platform trial for deceased organ donation optimisation: OPTIDON APT
Dashiell Gantner1,3,4,5, Alissa Higgins4,5, Helen Opdam2, Oliver Roydhouse5, Steve Webb4,5.
1Intensive Care, Alfred Health, Melbourne, Australia; 2Organ and Tissue Authority, Canberra, Australia; 3DonateLife Victoria, Melbourne, Australia; 4Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, Australia; 5Empiric Health, Melbourne , Australia
Background: Despite global efforts to improve deceased organ donation, there is substantial variability in donor- and organ- management practices, and hence organ utilisation. We propose a unified, international, multi-centre adaptive platform trial (APT) designed to efficiently evaluate and optimise pre- and post-retrieval interventions in deceased potential donors.
Objective: To develop and implement a scalable APT that improves the number and quality of organs successfully transplanted from deceased donors.
Methods: OPTIDON APT will use a pragmatic, Bayesian adaptive framework to evaluate pre- and post-retrieval interventions, grouped into domains based on intervention mechanism (e.g. pharmaceutical, artificial perfusion, donor blood pressure) and organ effect (e.g. hormonal resuscitation for heart donors, machine perfusion for individual organs). Such platform structures allow for new domains and/or interventions to be added and ineffective interventions dropped without starting new trials.
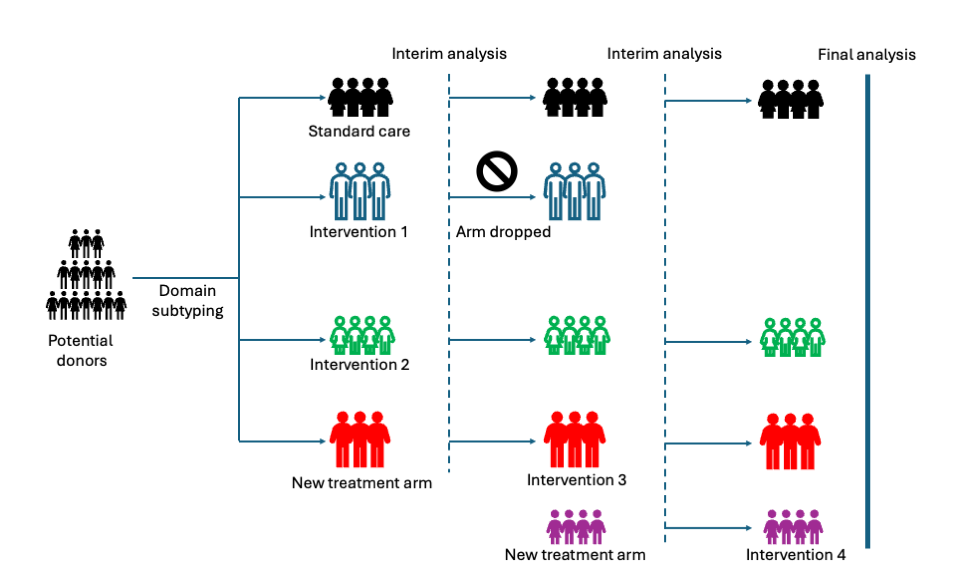
Randomisation can occur at the donor (pre-retrieval) and/or organ level (post-retrieval). The trial will leverage existing intensive care, donation, and transplant registries, including donor-recipient linkage. The primary outcome will be successful transplantation (number and function), with secondary outcomes including graft survival, donor metrics, and protocol adherence.
Collaboration and Governance: The platform will be globally scalable and coordinated through shared protocols, centralised analysis, and aligned outcome definitions. International partnerships will include donation and transplantation organisations and clinical trials networks across the UK, Canada, Spain, and Australasia. Governance will involve a trial steering committee, safety monitoring, and domain-specific expert groups. Local ethical standards would be applied for research in deceased donation and organ interventions.
Impact: This research methodology has the potential accelerate evidence generation in donor management, improve graft outcomes, and enhance international collaboration. The platform structure enables rapid adaptation to emerging interventions, supporting a sustainable path to evidence-based deceased donor optimisation.
[1] Donor management
[2] Organ optimisation
[3] Platform trials
[4] Bayesian trial design
[5] Multinational collaboration