DonorLines: A prospective cohort- and biobank study of deceased organ donors in the Netherlands
Belle Dielwart1,2, Anna M Posthumus2, Jip Jonker2, Marjolein Knoester3, Cyril Moers1, Henri G.D. Leuvenink1, Jan-Stephan F. Sanders2, Stephan J.L. Bakker2, Robert A. Pol1.
1Surgery, University of Groningen, University Medical Center Groningen, Groningen, Netherlands; 2Internal Medicine, division of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands; 3Virology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
DonorLines investigators.
Introduction: In recent years, the number of deceased organ donors has increased. However, this rise has been paralleled by an increase in mean donor age and greater use of extended criteria donors. These factors may negatively affect graft function, leading to increased morbidity and mortality in recipients. To improve deceased donor management, it is essential to identify potentially modifiable risk factors for poor transplant outcomes and to develop novel biomarkers that can predict organ quality. Despite these needs, research on deceased donors remains limited due to their dispersed geographic distribution, resulting in the loss of valuable data. As a new branch of the TransplantLines Biobank and Cohort study, DonorLines provides a comprehensive infrastructure to support high-quality clinical research in the field of organ donation and transplantation.
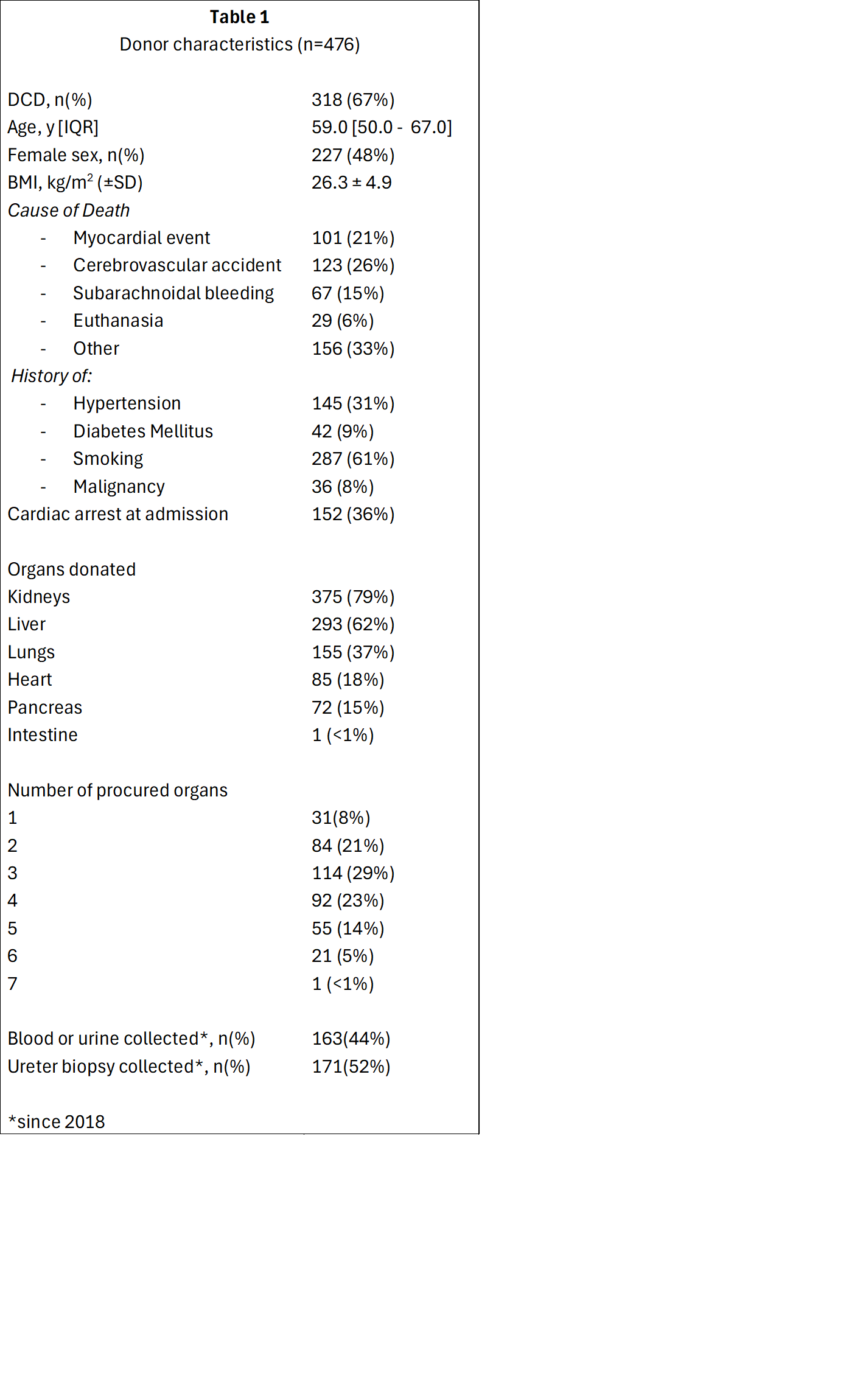
Method: All deceased organ donors >18 years with informed consent, are eligible for inclusion. Informed consent will be sought from the relatives in accordance with the applicable rules of the Dutch Transplant Foundation. Clinical data are collected during donor screening and procurement procedures, including donor characteristics, ICU treatment, and medical history. Biosamples, including blood, urine and various tissue types, are collected at donor screening and donor surgery and stored at -80˚C until future analysis
Results: To date, the study included 476 deceased (multi-) organ donors (Table 1), of whom 283 (59%) donated three or more organs. Median age was 59.0 [IQR: 50.0-67.0] years, 227 (48%) were female, and 318 (67%) donated after circulatory death. Since 2018, blood samples have been collected from 163 (44%) donors, and ureter biopsies have been obtained from 171 (52%) donors.
Conclusion: By systematically collecting and integrating clinical data, biomaterials and molecular profiles from deceased organ donors, DonorLines aims to facilitate the development of targeted strategies to improve the quality and viability of transplanted organs. The study is open for collaboration and data and materials are available upon request.
The TransplantLines Biobank and Cohort study was supported by grants from Astellas BV (project code: TransplantLines Biobank and Cohort study) and Chiesi Pharmaceuticals BV (project code: PA-SP/PRJ-2020-9136). The funders had no role in study design, data collection, analyses, or the decision to submit for publication. S.J.L.B. received grants from Chiesi Pharmaceuticals BV, Astellas BV and bioMerieux Benelux BV..
[1] Donor management
[2] Tissue banking
[3] Cohort study
[4] Biobank