Plasma dynamics of DAMPs and cf-mtDNA in controlled DCD lung transplantation: Association with primary graft dysfunction
Alberto Sandiumenge1, Ramon Marti5, Marina Perez-Redondo3, Maria Angeles Ballesteros2, Fernando Mosteiro4, Aroa Gomez-Brey1, Maria Jose Melia5, Elena Garcia Arumi5, Maria Deu6, Silvana Crowley8, Sara Naranjo7, Eva Fieira9, Victor Mora10, Irene Bello6.
1Donation and Transplantation Coordination, Vall Hebron University Hospital, Barcelona, Spain; 2Donation and Transplantation Coordination, Marques De Valdecilla University Hospital, Santander, Spain; 3Donation and Transplantation Coordination, Puerta De Hierro University Hospital, Madrid, Spain; 4Donation and Transplantation Coordination, A Coruña University Hospital, A Coruña, Spain; 5Vall D'hebron Research Institute, ,, Barcelona, Spain; 6Thoracic Surgery, Vall Hebron University Hospital, Barcelona, Spain; 7Thoracic Surgery, Marques De Valdecilla University Hospital, Santander, Spain; 8Thoracic Surgery, Puerta De Hierro University Hospital, Madrid, Spain; 9Thoracic Surgery, A Coruña University Hospital, A Coruña, Spain; 10Pneumology, Marques De Valdecilla University Hospital, Santander, Spain
Background: Controlled donation after circulatory determination of death (cDCD) has become a widely used strategy to expand the lung donor pool. Despite favorable outcomes, concerns exist about the effects of warm ischemia time (WIT) on the lungs, that may experience hypoxia, metabolic stress, and mitochondrial dysfunction, potentially leading to ischemia-reperfusion injury (IRI) and systemic release of damage-associated molecular patterns (DAMPs), particularly cell-free mitochondrial DNA (cf-mtDNA). These molecules have been linked to innate immune activation and complications such as primary graft dysfunction (PGD). While molecular injury patterns in cDCD lungs are known, it remains unclear whether these changes are reflected systemically and how they relate to clinical outcomes.
Methods: We conducted a prospective, multicenter, observational study in four Spanish transplant centers. Adult recipients of single or bilateral lung transplantation (LTx) from cDCD and brain-dead donors (DBD) were matched 1:1 by age, sex, and indication. Blood samples were collected from donors (before skin incision and before perfusion) and from recipients (before implantation, immediately after reperfusion, and 72 hours post-transplant). Plasma cf-mtDNA levels were measured and analyzed by donor type, PGD, and early mortality (≤3 months).
Results: We included 80 LTx recipients (40 cDCD, 40 DBD). Baseline demographic and clinical characteristics were similar, except for more corticosteroid and vasoactive use in DBD donors. Donor cf-mtDNA levels were comparable between cDCD and DBD at both sampling points, suggesting WIT does not lead to greater systemic mitochondrial injury before organ retrieval. In recipients, a delayed increase in cf-mtDNA was observed at 72 hours post-transplant in the cDCD group, but not immediately after reperfusion, indicating a delayed systemic response (Figure1). Higher cf-mtDNA levels in donor plasma were associated with PGD in recipients, though this was not reflected in recipient plasma at any time. PGD incidence and early mortality were similar between groups.
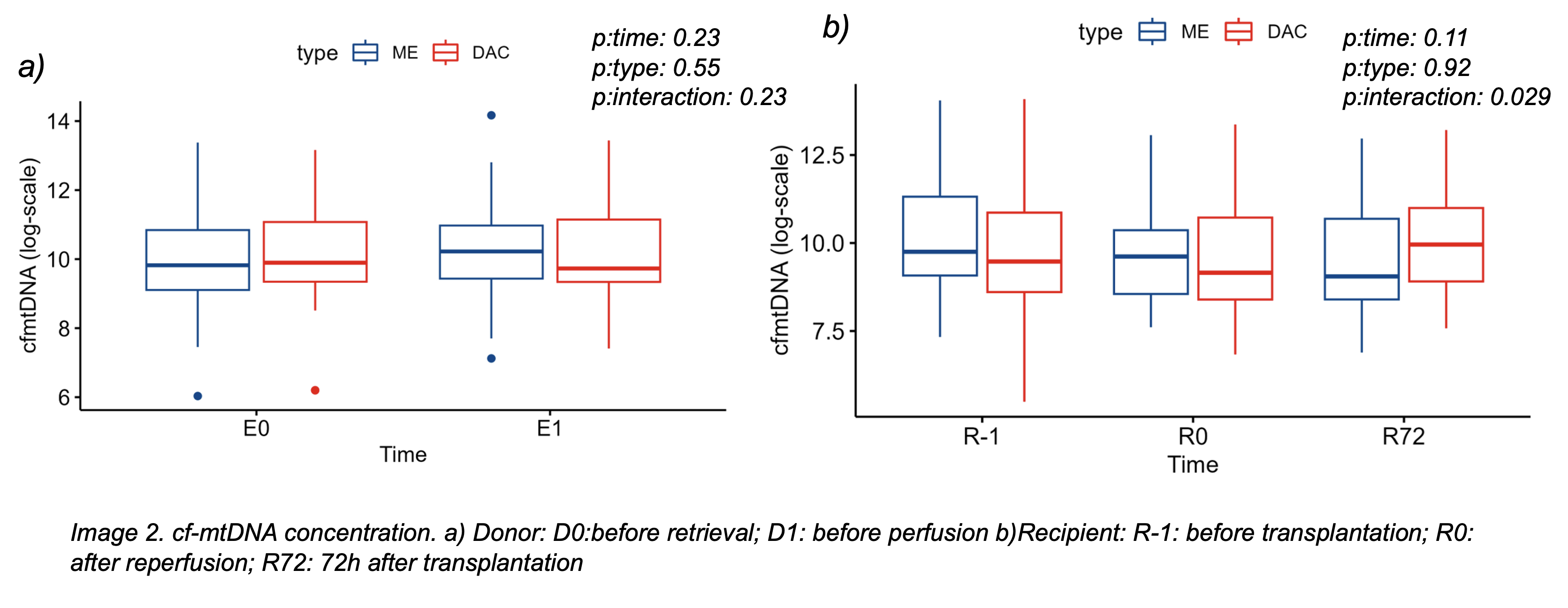
Conclusions: cDCD lung donors do not show increased systemic mitochondrial damage markers cf-mtDNA at procurement, but their recipients develop a delayed rise in cf-mtDNA post-transplantation. This suggests WIT-related injury may present subacutely. Donor cf-mtDNA may serve as a pre-implantation biomarker of PGD risk. Overall, cDCD is a safe, effective donor source, and these molecular insights may help refine graft evaluation.
Fundacion Mutua Madrileña.
[1] Controlled donation after circulatory determination of death
[2] donation after neurological determination of death
[3] inflamatory response
[4] damage-associate molecular patterns
[5] free mithocondrial DNA
[6] lung donation and trasplantation
[7] Primary graft disfunction
[8] mortality