Implementing donation after circulatory determination of death practise to Finland
Maarit Lång1, Hilja-Maaria Stauffer1, Jaakko Långsjö2, Kirsi Rantanen3, Aki Uutela4, Kaisa Ahopelto4, Ilkka Helanterä4, Marko Lempinen4, Arno Nordin4.
1Joint Authority Administration, Organ Donation, HUS, Helsinki, Finland; 2Intensive Care Unit, TAYS, Tampere, Finland; 3Neurology, HUS, Helsinki, Finland; 4Transplantation and Liver Surgery Unit, HUS, Helsinki, Finland
Introduction: The Finnish National Action Plan on Organ Donation and Transplantation aims to ensure equitable and timely access to transplantation for all patients medically indicated for this treatment. To achieve this goal, approximately 40 deceased organ donors per million population (pmp) are required. Until 2021, organ donation in Finland was limited exclusively to brain dead donors, yielding a donor rate of approximately 19–25 dead donors pmp. As the availability of organs directly constrains transplantation rates, donation after circulatory determination of death (DCDD) has been introduced as a complementary pathway alongside the existing brain death donation program to expand the donor pool.
Methods: This objective follow-up study evaluates the development of DCDD in Finland and assesses the quality of kidney transplantation utilizing the DCDD pathway. The donors were under 70 yrs. DCDD was limited to kidney procurement and transplantation only.
Results: Between 2021 and 2024, the number of deceased donors increased from 21.5 to 27.6 pmp. The rate of brain-dead donors remained stable, ranging from 20.8 to 22.1 pmp during the implementation of DCDD. Kidney transplantations increased by 20% over the four-year period. The proportion of DCDD donors among all deceased organ donors from September 2021 to May 2025 is depicted in Figure 1.
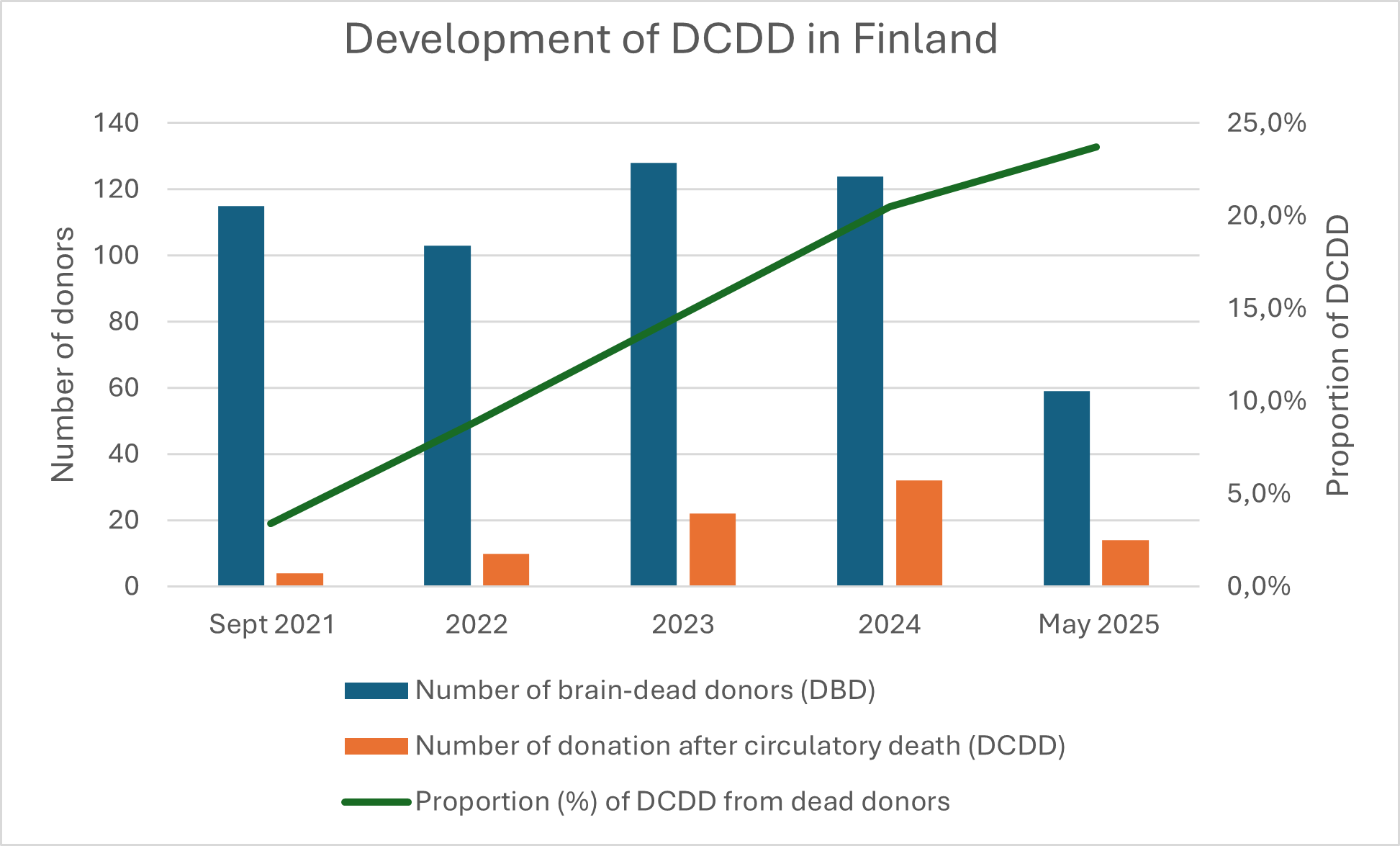
The mean age of DCDD donors was 53.3 years (standard deviation [SD] 11.8), with 31% (23/74) aged over 60 years and 70% (52/74) male. The mean time from treatment withdrawal to circulatory arrest was 31 minutes (min) (range 2 min to 2 hours (h) 58 min). Super rapid retrieval technique was used for procurement. Mean cold ischemia time for kidneys was 8 h 4 min (range 2 h 47 min to 14 h 53 min); hypothermic machine perfusion was employed in majority of cases.
Graft function over a two-year period, expressed as mean (95% confidence interval, CI), is presented in Figure 2.
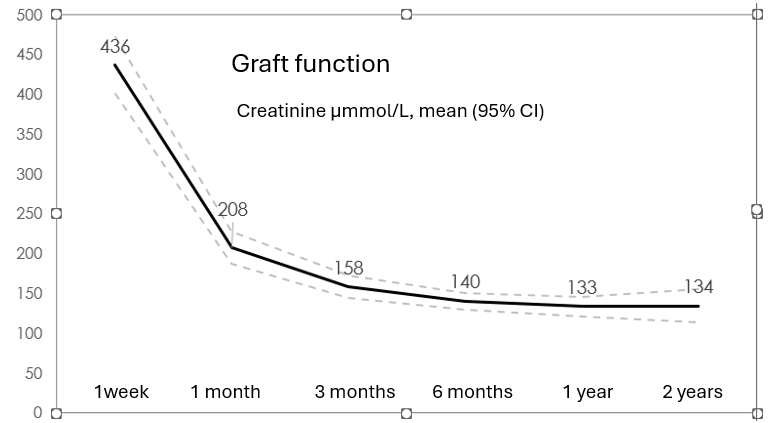
Early graft function was seen in 22% (29/134) of transplants. Slow graft function, defined as serum creatinine > 265 µmol/L on postoperative day 5, was observed in 36% (48/134). The incidence of delayed graft function, (need for dialysis during the first postoperative week) was 40% (53/134). Nonfunctioning grafts accounted for 3% (4/134). At six months, graft function was significantly poorer in donors over 60 years compared to younger donors (creatinine 160 ± 57 vs. 129 ± 48 µmol/L, p < 0.05), although no significant difference was noted at one year (148 ± 63 vs. 126 ± 51 µmol/L, n.s.).
Conclusion: The integration of DCDD into Finnish organ donation practice has been successful. Kidney transplantation outcomes from DCDD donors are favorable, and the overall number of kidney transplants has increased. Future perspectives include the implementation of normothermic regional perfusion to enhance both graft quality and the quantity of transplantable organs.
Maarit Lång.
[1] Donation after circulatory determination of death
[2] graft function
[3] deceased organ donation
[4] kidney transplantation